Answer 1 of 7. It can predict the shape of nearly all compounds that have a central atom as.

7 4 Molecular Structure And Polarity Chemistry Libretexts
The theory was first presented by Sidgwick and Powell in 1940.

. The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an. Subscripts tell us how many of a type of atom there areEx. The shape of a molecule is determined by the location of the nuclei and its electrons.
What does the VSEPR theory postulate. 2 BP and 3 LP. Steps for using VSEPR Theory-draw a lewis dot diagram-predict the geometry around the central atom-name the molecular shape.
20 Why is the shape of receptors important. The VSEPR theory assumes that each. Valence shell electron pair repulsion theory vesper for short classifies molecules on the basis of the number of electron pairs bonding and lone pairs associated with the central atom.
VSEPR theory says that the shapes of molecules are determined by the repulsion of both bonding and non-bonding outer electrons. 4 BP and 1 LP. See this older answer.
The electrons and the nuclei settle into positions that minimize repulsion and maximize attraction. The VSEPR Theory Valence Shell Electron Pair Repulsion Theory is based on the fact that there is a repulsion between the pairs of valence electrons in all the atoms and the atoms will always try to arrange themselves in a manner in which the electron pair repulsion is minimized. VSEPR stands for Valence Shell Electron Pair Repulsion.
The steric number is the sum of bonds and lone pairs on the central atom in a Lewis dot structure. 17 What does the VSEPR theory predict molecular shape apex. Advertisement Advertisement correatp0525 correatp0525 Answer.
VSEPR models are based on the concept that electrons around a central atom will configure themselves to minimize repulsion and that dictates the geometry of the molecule. The VSEPR theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. There is no direct relationship between the formula of a compound and the shape of its molecules.
The VSEPR theory is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized. VSEPR Theory - Molecular Geometry. The shapes of molecules can be predicted from their Lewis structures by using a model developed about 30 years ago known as the Valence Shell Electron Pair Repulsion VSEPR theory-The VSEPR concept revolves around the idea that the electrons in a molecule repel each other and will try to get as far away from each other as possible.
Valence shell electron pair repulsion theory VESPER for short. The lone pair occupies space around nitrogen atom just as the bonding pairs do what does the shape of the molecule refer to. Using the VSEPR theory the electron bond pairs and lone pairs on the center atom will help us predict the shape of a molecule.
2 H2O 2 H2Os 2x2 Hydrogens 2x1 Oxygen. The shapes of these molecules can be predicted from their Lewis structures however with a model developed about 30 years ago known as the valence-shell electron-pair repulsion VSEPR theory. The central carbon atom is still joined to two other atoms and the electron clouds that connect the two oxygen atoms are 180 apart.
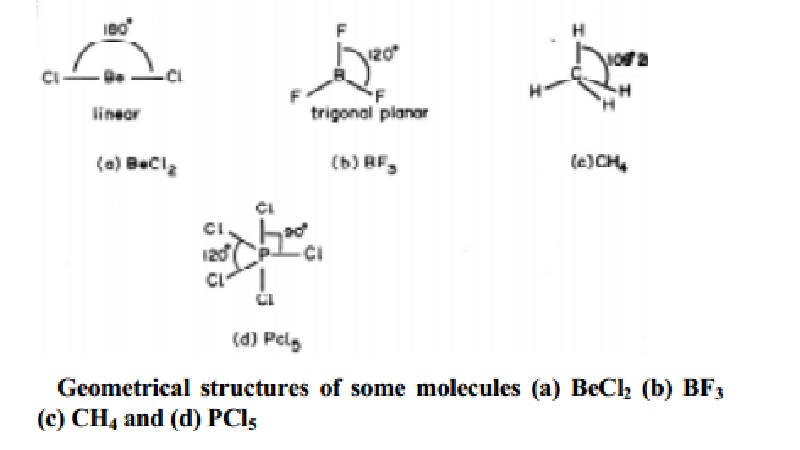
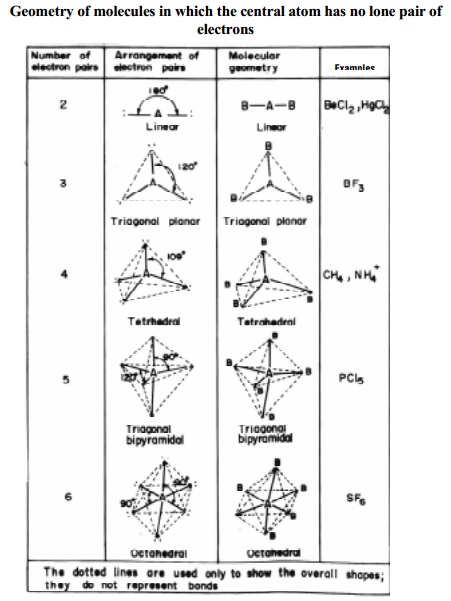
Linear molecule A simple triatomic molecule of the type AX2 has its two bonding orbitals 180 apart producing a molecule of linear geometry. According to vsepr theory the repulsion between electron pairs causes molecular shapes to adjust so that the valence electron pairs stay as far apart as possible are unshared pairs important in predicting the shapes of molecules. VSEPR counts the number of electron pairs around a central atom both bonding and non-bonding and assigns a shape according to the Platonic solids.
The shape of a molecule. H2O 2 Hydrogens 1 OxygenCoefficients tell us how many of the molecule there areEx. This makes no difference to VSEPR theory.
VSEPR is a system for predicting the structure of a molecule based on the steric number of the molecules central atom. Thus carbon dioxide is. BP is Bond Pair and LP is Lone Pair.
Valence Shell Electron Pair Repulsion Theory. A P E X. 4 BP and 2 LP.
It states that electron pairs in the valence shell of an atom repel each other. 21 What would cause. VSEPR - Valence shell electron pair repulsion theory predicts the shape of a molecule.
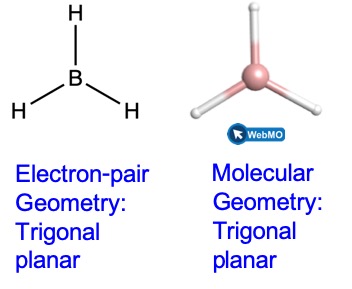
For example the molecule CH4 has a tetrahedral shape because thats. This arrangement of the atom shapes the geometry of the resulting molecule. Valence shell electron-pair repulsion theory VSEPR theory enables us to predict the molecular structure including approximate bond angles around a central atom of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure.
It is a fact that electron pairs arranged around a. The electrons are attached. You can also predict _____ _____ and _____ _____ by using VSEPR Theory.
2 BP and 1 LP. Advertisement Advertisement New questions in Chemistry. 18 Why do we study molecular shape.
The shape of a molecule is determined by the repulsion of electron pairs. Predicting the Shapes of Molecules. Terms in this set 20 bent.
19 Why is it important to know the bonding and the shape of the molecules.
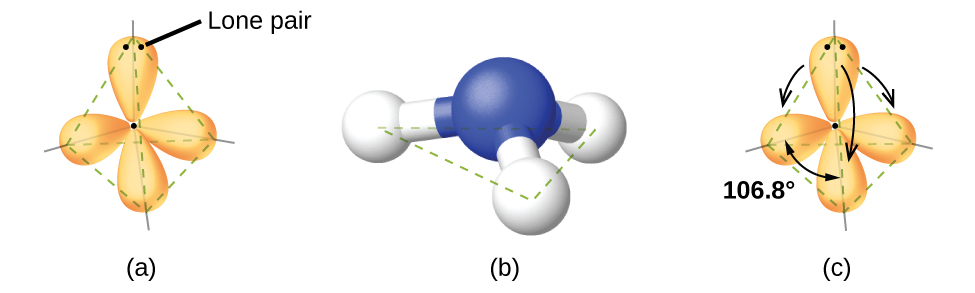
7 6 Molecular Structure And Polarity Chemistry
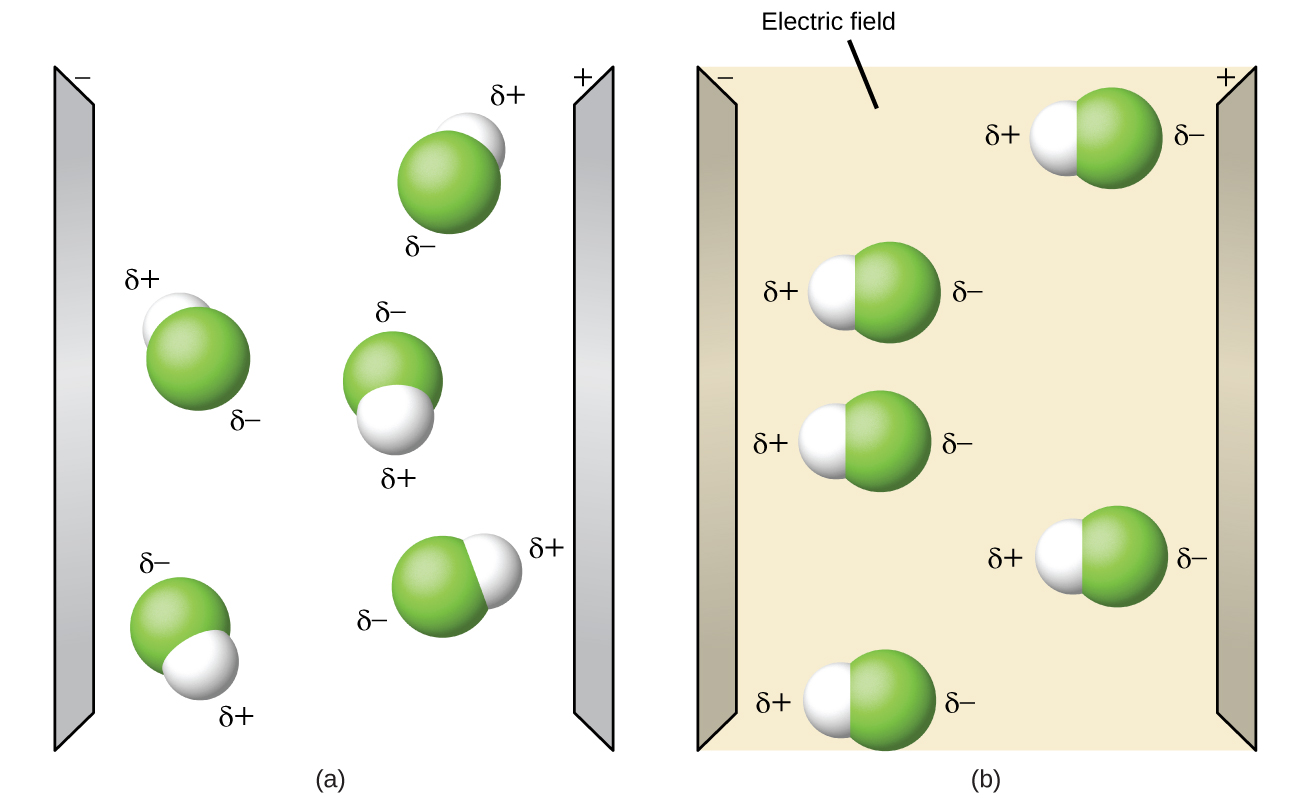
7 6 Molecular Structure And Polarity Chemistry
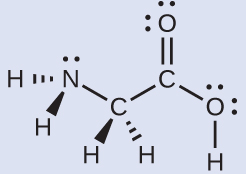
Molecular Structure And Polarity Chemistry Atoms First 2e

6 3 Molecular Shape Introductory Chemistry

Molecular Geometry And Covalent Bonding Models Molecular Geometry Teaching Chemistry Chemistry Basics

6 3 Molecular Shape Introductory Chemistry
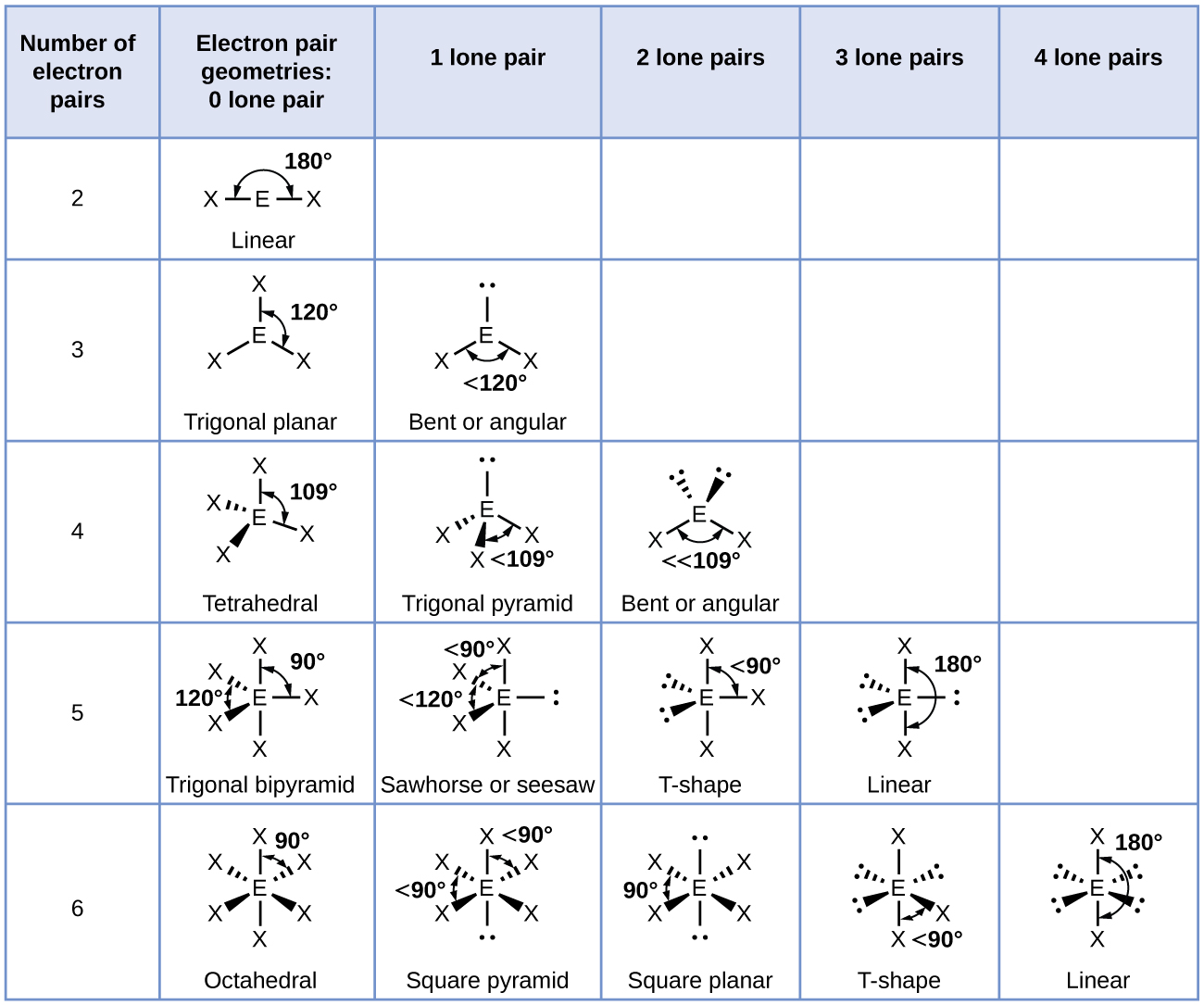
7 6 Molecular Structure And Polarity Chemistry

What Does The Vsepr Theory Predict A The Chemical Formula Of A Molecule B The Size Of A Brainly Com
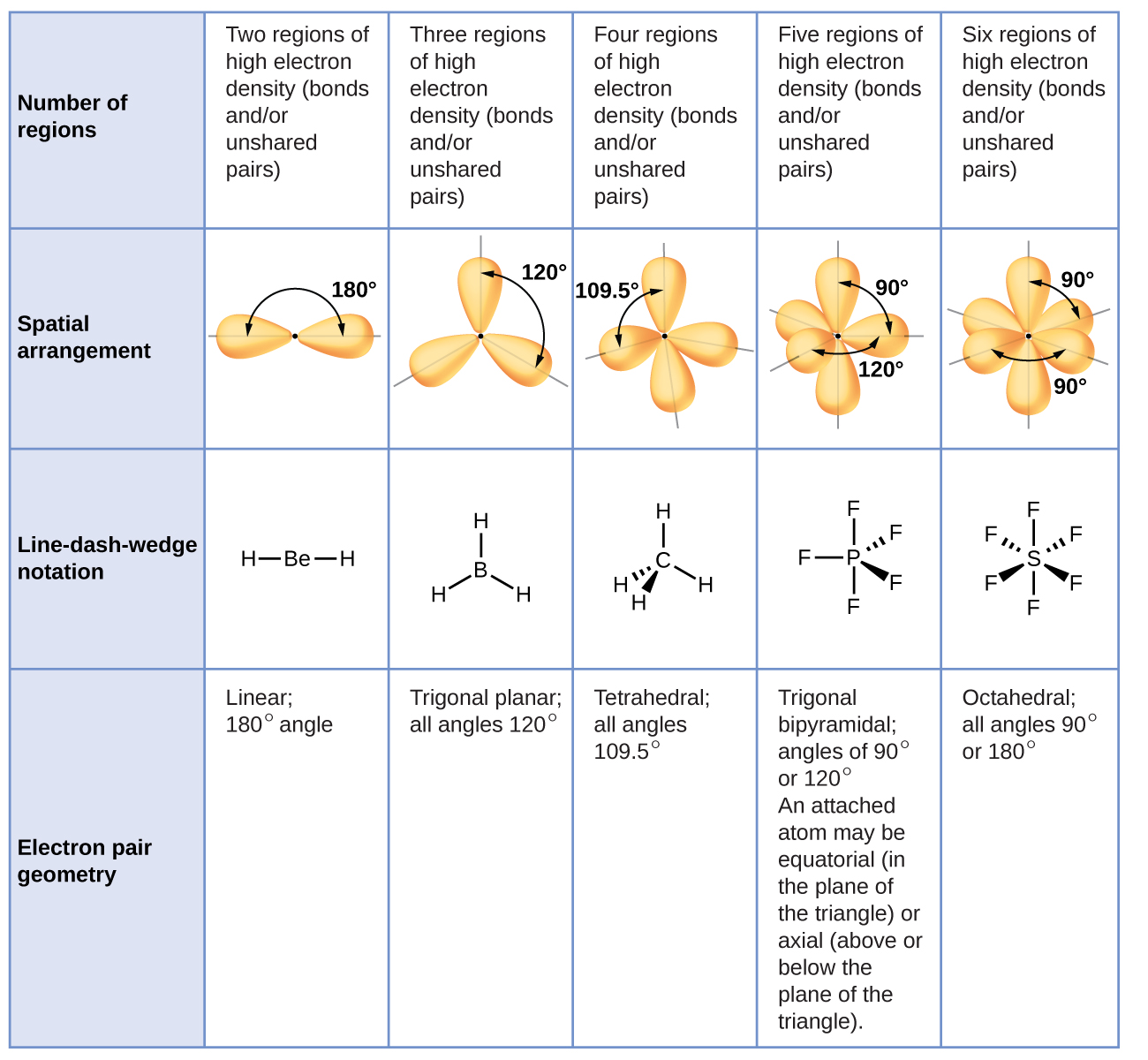
7 6 Molecular Structure And Polarity Chemistry
Why Does Every Molecule Have Definite Geometry Quora
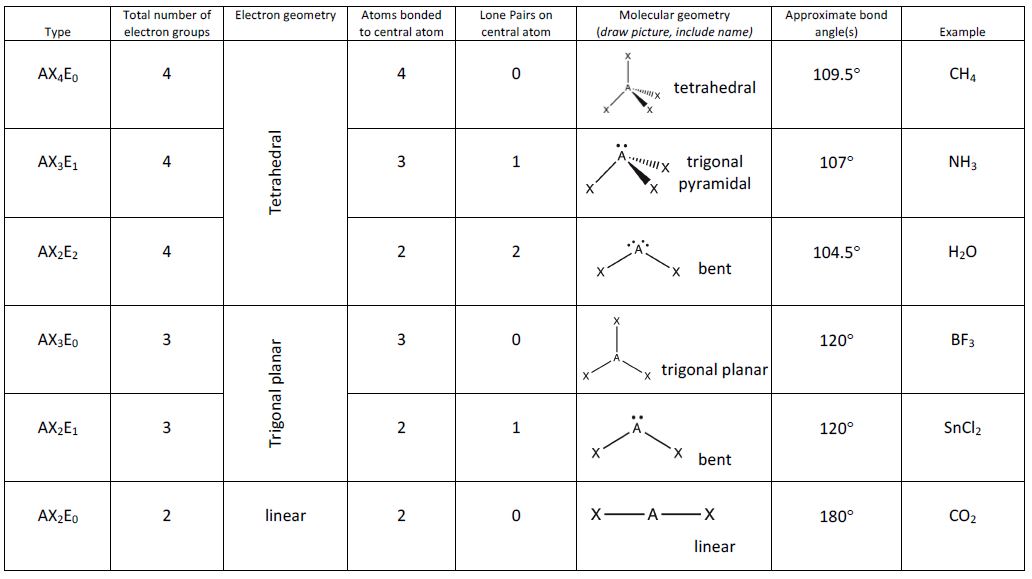
Predicting Molecular Shapes Vsepr Model M9q1 Uw Madison Chemistry 103 104 Resource Book

Molecular Structure And Polarity Chem 1305 Introductory Chemistry
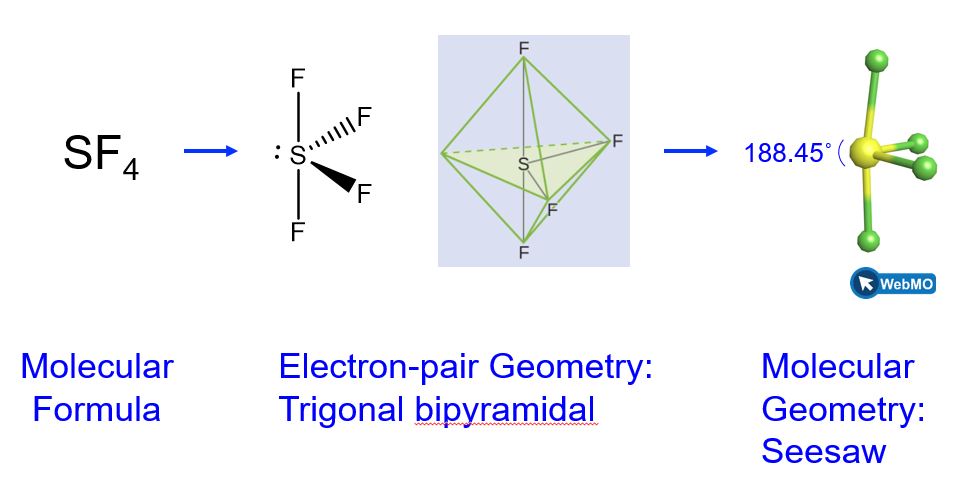
Predicting Molecular Shapes Vsepr Model M9q1 Uw Madison Chemistry 103 104 Resource Book
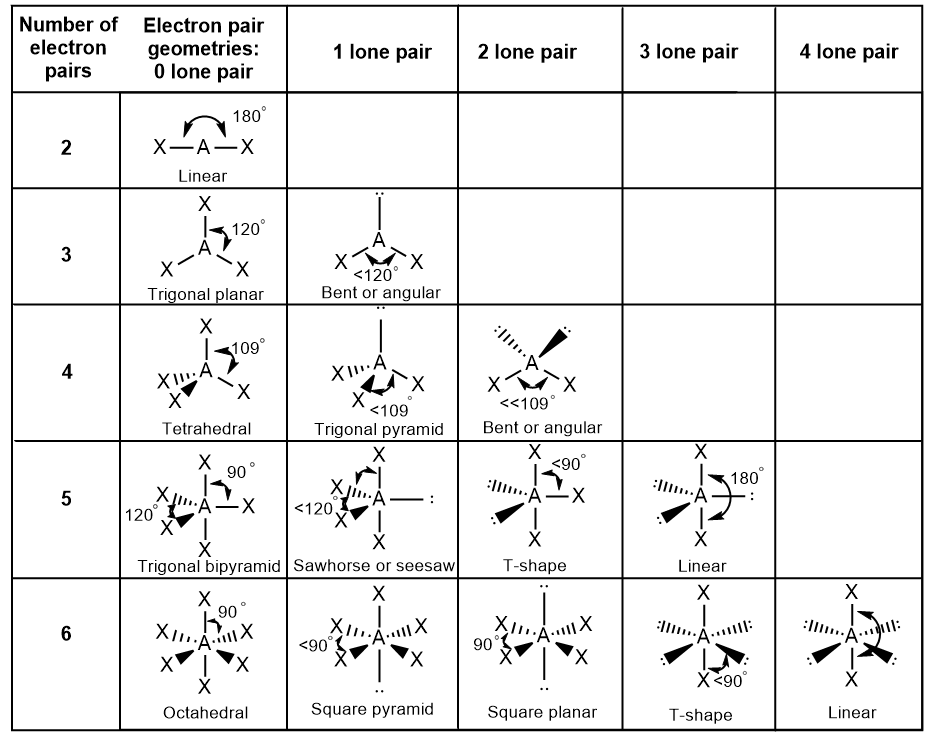
9 5 Vsepr General Chemistry For Gee Gees
Valence Shell Electron Pair Repulsion Theory Vsepr Theory
Predicting Molecular Shapes Vsepr Model M9q1 Uw Madison Chemistry 103 104 Resource Book
Valence Shell Electron Pair Repulsion Theory
Valence Shell Electron Pair Repulsion Theory Vsepr Theory
